 Designer bacteria to treat symptoms such as constipation and diarrhea
Designer bacteria to treat symptoms such as constipation and diarrhea
The Gut Microbiome Lab's research on how gut microbiota and their metabolites influence motility could help scientists develop new targeted therapies for people with gastrointestinal disorders such as irritable bowel syndrome.
Impact of Gut Microbiota on Host Physiology
The Gut Microbiome Lab at Mayo Clinic is studying the impact of microbial colonization on host physiology. As a part of this project, Dr. Kashyap's research team is exploring how gut microbiota and their metabolites influence intestinal secretion, motility and pain sensation, such as in irritable bowel syndrome, which is a disorder of the gut-brain axis.
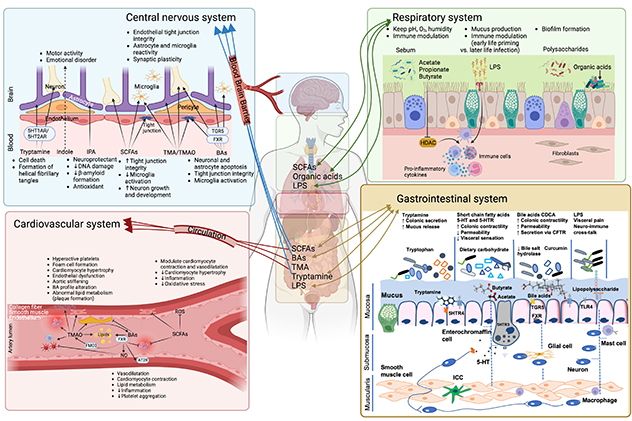 Effects of gut microbiota on host physiology
Effects of gut microbiota on host physiology
This illustration provides a comprehensive overview regarding the effects of gut microbiota on host physiology and factors that influence gut microbiota composition in health and disease.
Irritable bowel syndrome is a common condition that affects approximately 11% of the global population. Changes in stool form and frequency, accompanied by abdominal discomfort or pain, characterize this syndrome.
Irritable bowel syndrome is categorized into subtypes based on predominant symptoms: constipation (IBS-C), diarrhea (IBS-D) or mixed symptoms (IBS-M). Despite its prevalence, the pathophysiology of irritable bowel syndrome is complex, and targeted therapeutic options remain limited. Emerging evidence highlights the role of gut microbial metabolites in maintaining gastrointestinal function, but how these metabolites influence irritable bowel syndrome pathophysiology remains poorly understood.
The intestinal lumen, the largest and most diverse repository of bioactive compounds in the body, is generated through bacterial metabolism. These bacterial metabolites play critical roles in local and systemic physiology; yet, the mechanisms by which they are sensed, decoded and translated by the host remain incompletely understood.
Given the vast array of bacterial metabolites — far outnumbering the genetically encoded receptors in the host — it is likely that noncanonical ligand-receptor interactions mediate many of these responses. Understanding these complex interactions is essential for deciphering the role of microbial metabolites in health and disease.
Recent findings from Dr. Kashyap's Gut Microbiome lab have revealed key insights into these processes. For example, the lab found that gut bacteria and their metabolites modulate gastrointestinal physiology by increasing serotonin production from the enterochromaffin cells and altering the expression of serotonin receptor subtypes in the gastrointestinal tract.
Serotonin is an important neurotransmitter and paracrine messenger in the gastrointestinal tract. It plays a critical role in regulating gastrointestinal physiology in health and disease. The lab discovered that tryptamine, a microbial metabolite, enhances secretion and mucus release via serotonin receptor pathways, accelerates gastrointestinal transit, and protects against inflammation. Elevated tryptamine levels in patients with IBS-D highlight its clinical relevance. In IBS-C, multi-omics analyses have shown consistent reductions in stool metabolites such as hypoxanthine and butyrate.
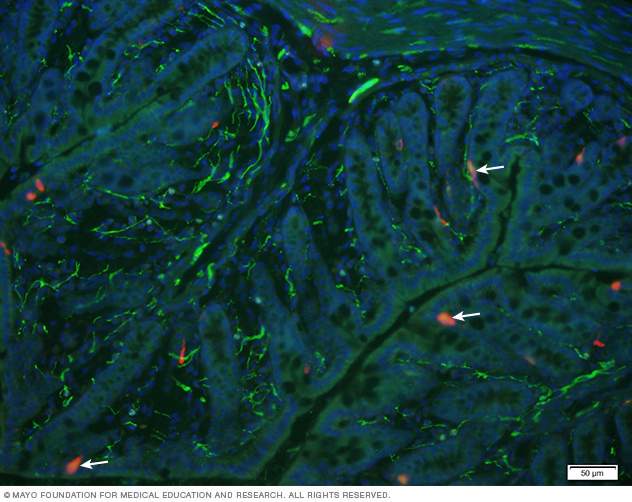 Enterochromaffin in the colonic mucosa
Enterochromaffin in the colonic mucosa
This image illustrates presence of enterochromaffin cells (red) colocalized with serotonin receptor (highlighted by arrows) in the colonic mucosa. DAPI was used to localize epithelial nuclear staining.
To advance the understanding of irritable bowel syndrome, the lab is investigating how different microbial metabolites and interactions among these metabolites influence the molecular pathways underlying irritable bowel syndrome pathophysiology. Using innovative approaches such as organoid models, advanced imaging, transcriptomics, epigenomics and gnotobiotic mouse models, the lab aims to uncover how metabolites — individually and together — impact gastrointestinal transit and function. By deciphering these complex interactions, the lab's research seeks to establish the groundwork for developing novel, mechanism-based microbial therapies for irritable bowel syndrome. These therapies could offer more effective and targeted treatment options for this challenging and widespread condition.
 Calcein-acetoxymethyl labeled murine colonoids
Calcein-acetoxymethyl labeled murine colonoids
Colonoids