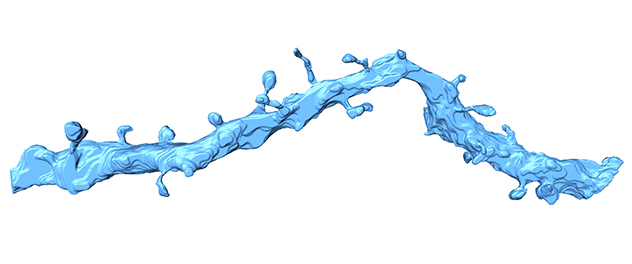
 3D neuronal dendrite in the brain
3D neuronal dendrite in the brain
This high-resolution 3D image has been reconstructed from a prefrontal rodent brain using a serial block-face electron microscope and Amira software.
Projects
Basal ganglia circuits, astrocyte-neuron interaction and alcohol use disorder
Maladaptive shifts from goal-directed to habitual actions may lead to severe psychopathologies, such as obsessive-compulsive disorder, impulsivity and addiction. Persistent habitual reward-seeking, characterized by insensitivity to reversal of action-outcome contingency, even after reward devaluation, is a common feature of addiction. The dorsal striatum has a critical role in shaping goal-directed and habitual actions, which are the main determinants of the reward-dependent decision-making process. Specifically, the caudatelike dorsomedial striatum (DMS) and putamenlike lateral striatum (DLS) neuronal populations are responsible for goal-directed behavior.
Astrocytic processes near the synaptic milieu clear glutamate, which protects neurons from excitotoxicity. Also, astrocytes regulate other neurotransmitters in the synaptic cleft and involve a wide variety of gliotransmission events. Our recent studies revealed that chemogenetic activation of DMS astrocytes increases and enhances the activities of indirect medium spiny neurons, projecting to external globus pallidus (GPe). We also examined the role of astrocytes in the GPe. Both chemogenetic astrocyte activation of DMS and GPe promote the transition from habitual to goal-directed reward-seeking behaviors.
Chemogenetic activation of GPe astrocytes suppressed neuronal firing in the GPe, thereby promoting goal-directed reward-seeking behavior. Consistently, increased GPe astrocytic activity reduced habitual reward-seeking, suggesting a potential mechanism to diminish ethanol-seeking and drinking behaviors. Also, we found that the astrocytic GABA transporter 3 (GAT3) plays a critical role in regulating astrocyte activity and alcohol-seeking behaviors. Furthermore, our recent study shows that caspase-induced partial ablation of DLS indirect medium spiny neurons projecting to the GPe increases compulsive ethanol-seeking and drinking behaviors.
Within the GPe, parvalbumin-expressing neurons, which constitute over half of GPe neurons, integrate inhibitory inputs from indirect medium spiny neurons in the dorsal striatum. Also, we found that partial ablation of forkhead box p2 (FOXP2)-expressing arkypallidal neurons (GPe→DLS) facilitates a shift from goal-directed to habitual reward- and alcohol-seeking behaviors.
Currently, we aim to:
- Investigate how striatal astrocyte activation and inhibition alters behaviors such as compulsive drug and alcohol seeking and taking.
- Determine whether astrocyte and neural activities are associated with alcohol and other addictions.
- Examine how circuit-specific brain stimulation is translationally treatable for alcohol and other addictions.
Our research will advance molecular understanding of alcohol use disorder at anatomical and circuital levels and provide an integrated tool to identify and validate novel candidate targets to treat alcohol use disorder. This knowledge could be applied to other behavioral and neuropsychiatric disorders.
Alcohol-induced neuroinflammation and Alzheimer's disease
Alcohol use disorder (AUD) has been associated with the development of neurodegenerative diseases, including Alzheimer's disease. Our recent retrospective analysis with 6,036 subjects, including 269 AUD+ subjects, indicates that AUD+ was significantly associated with lower scores of cognition and memory function relative to people with AUD.
On the other hand, some studies demonstrate that moderate alcohol consumption may protect against dementia and cognitive decline. Recently, we examined astrocyte function, low-density lipoprotein (LDL) receptor-related protein 1 (LRP1), and the NF-κB p65 and IKK-α/β signaling pathways in modulating neuroinflammation and amyloid-beta (Aβ) deposition. We assessed apolipoprotein E (ApoE) in the brain of APP/PS1 mice using immunohistochemistry and enzyme-linked immunosorbent assay in response to low to moderate ethanol exposure.
Our findings demonstrate that moderate ethanol exposure reduced astrocytic glial fibrillary acidic protein (GFAP) and ApoE levels in the cortex and hippocampus in presymptomatic APP/PS1 mice. Increased LRP1 protein expression was accompanied by dampening the IKK-α/β-NF-κB p65 pathway, resulting in decreased IL-1β and TNF-α levels in male mice. Notably, female mice show reduced levels of anti-inflammatory cytokines IL-4 and IL-10 without altering IL-1β and TNF-α concentrations. In both males and females, Aβ plaques, a hallmark of Alzheimer's disease, were reduced in the cortex and hippocampus of APP/PS1 mice exposed to ethanol starting at the presymptomatic stage.
Consistently, moderate ethanol exposure increased FDG-PET-based brain activities and normalized cognitive and memory deficits in the APP/PS1 mice. Our findings suggest that activating LRP1 expression and function potentially reduces neuroinflammation and attenuates Aβ deposition. Our study implies that reduced astrocyte-derived ApoE and LDL cholesterol levels are critical for attenuating Alzheimer's disease pathology.
Based on these findings, we aim to:
- Investigate whether an LRP1 agonist reduces neuroinflammation and cognitive decline in humanized ApoE4 knockin mice.
- Determine whether astrocyte-microglia interaction is critical for alcohol-induced inflammation and cytokine-dependent Alzheimer's disease.
- Develop clinically applicable neuroimaging biomarkers, such as FDG-PET in animal models.
Our study implies that reduced astrocyte-derived ApoE and LDL cholesterol levels are critical for attenuating Alzheimer's disease pathology.
Biomarkers associated with alcohol use disorder and psychiatric diseases
Our previous findings show that the nonsynonymous 647T/C variant of ENT1 that replaces isoleucine with threonine at transmembrane domain 6 is associated with a higher predisposition to alcohol withdrawal seizures in people with alcohol use disorder. Functional analysis demonstrated that prolonged ethanol exposure increased uptake activity of ENT1-216Thr both for inosine and adenosine, compared with ENT1-216Ile. Our findings reveal the important role of a genetic variant of ENT1 in developing alcohol withdrawal-induced seizures in humans and animal models.
Our laboratory has established glutamate and other brain metabolites, such as glutamate, acetyl aspartate, choline, inositol compounds and total creatine, in both the anterior cingulated and the ventral striatum. Using in vivo 7-tesla (300 megahertz) and 16.5-tesla (700 megahertz) magnetic resonance spectroscopy, we found that the levels are altered when comparing the basal level to that during an alcohol withdrawal period.
Recently, using proteomics and metabolomics of human serum and cerebrospinal fluid samples, we identified several biomarkers associated with pharmacological treatments and symptom severity and improvements.
Collaborating with clinical investigators at Mayo Clinic, we aim to:
- Identify genetic, imaging, proteomics and metabolomics biomarkers associated with alcohol use disorder.
- Develop neurotechnologies to treat alcohol use disorder and neuropsychiatric disorders.
- Integrate biomarkers and clinical data to implement clinically useful treatment modalities.
We believe that our research investigating genetic predispositions and integration of muti-omics biomarkers is crucial to developing precise treatments for alcohol use disorder and other neuropsychiatric disorders.