Focus Areas
The Phospholipid Signaling in Embryonic Development and Human Diseases Lab studies the roles of phosphoinositide species and enzymes in various cellular processes, such as cell migration, vesicular trafficking and ion channel activity. Dr. Ling's team is interested in how these phospholipids participate in developmental events, tissue and organ homeostasis, and related human diseases.
Epithelial-to-mesenchymal transition and cancer metastasis
The epithelial-to-mesenchymal transition is a key event for typical embryonic development, wound healing and tissue regeneration, as well as organ fibrosis and cancer metastasis. Via this highly dynamic process, epithelial cells convert into mesenchymal phenotypes, becoming less differentiated and more migratory and invasive. Morphologically, the epithelial-to-mesenchymal transition starts from the disassembly of cell-cell adhesion and loss of epithelial polarity, and then active assembly of dynamic cell-matrix adhesions and migration.
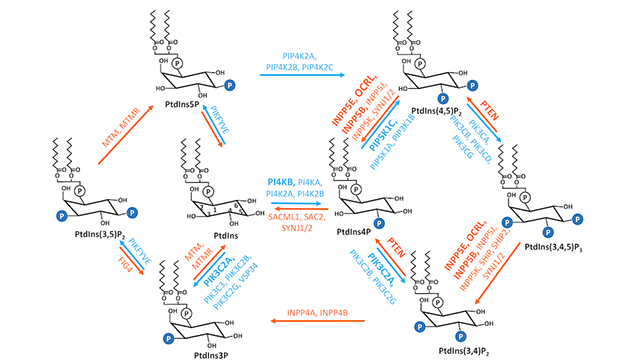 Interconversions of 7 phosphoinositide metabolites
Interconversions of 7 phosphoinositide metabolites
Phosphoinositides, reversibly phosphorylated derivatives of the membrane phosphatidylinositol, are low abundant secondary messengers. They serve as key identity determinants of various cellular membrane compartments and act as spatiotemporal cues to direct signaling events. As such, phosphoinositides are indispensable to the integrity of cells.
Phosphatidylinositol-4,5-bisphosphate, abbreviated as PtdIns(4,5)P2, is a key regulator of cell adhesions and the actin cytoskeleton. Besides functioning as a substrate of phospholipase C (PLC) and phosphoinositide 3-kinase (PI3K) to influence signaling events in the epithelial-to-mesenchymal transition. PtdIns(4,5)P2 directly regulates the epithelial-to-mesenchymal transition from multiple aspects, including gene transcription, protein stability, targeted vesicular trafficking and the interaction between adhesion proteins.
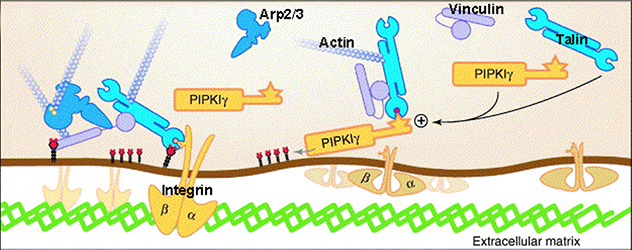 Phosphatidylinositol-4,5-bisphosphate regulates the assembly of focal adhesions and cell migration
Phosphatidylinositol-4,5-bisphosphate regulates the assembly of focal adhesions and cell migration
Phosphatidylinositol-4,5-bisphosphate binds to talin and promotes the interaction of talin with integrin.
The lab's team and others have observed that Type I gamma phosphatidylinositol phosphate kinase (PIPKIγ), the dominant generator of PtdIns(4,5)P2, directly binds to multiple structural and signaling molecules to support these processes. The intent of our lab is to understand the underlying molecular mechanisms and molecules that can be targeted to manipulate these important life events.
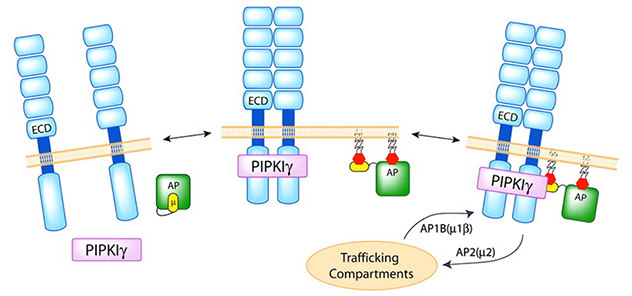 Phosphoinositide signaling regulates the polarized vesicular trafficking
Phosphoinositide signaling regulates the polarized vesicular trafficking
Type I gamma phosphatidylinositol phosphate kinase (PIPKIγ) specifically targets to, and creates a pool of, phosphatidylinositol-4,5-bisphosphate at the nascent adherence junctions. This serves to guide the directed deposition of E-cadherin and facilitate the maturation of adherence junctions and epithelial polarization.
Trafficking of proteins and lipids in primary cilia
Although largely considered a vestige before 1999, the primary cilium is now demonstrated as a signaling center to interpret both chemical and mechanical signals. Major components of multiple signaling pathways specifically localize in primary cilia. These include pathways that are essential for the biogenesis and homeostasis of tissues and organs, such as signaling mediated by hedgehog, Wnt, G protein-coupled receptors and growth factor receptors. Although continuously extended from the plasma membrane, the ciliary membrane possesses unique composition and compartmentalization of phosphoinositides.
PtdIns4P and PtdIns(4,5)P2 are the most well-studied ciliary phosphoinositides. PtdIns4P is the dominant phosphoinositide along the ciliary membrane. Whereas PtdIns(4,5)P2 is mainly limited to the proximal part of the cilium and the ciliary base. It has been suggested that the unique compartmentalization of phosphoinositide species, kinases and phosphatases in different sections of cilia is critical for the regulated trafficking of signaling proteins into and out of cilia. In particular, phosphoinositide signaling plays an important role in the assembly and maintenance of the diffusion barrier at the base of cilia.
The lab's current efforts focus on dissecting the phosphoinositide-dependent ciliary trafficking machinery using biochemistry and cell biological approaches, such as spatial interactome analysis and super-resolution microscopy.
Polycystic kidney disease
Polycystic kidney disease (PKD) — a group of genetic conditions with progressive renal cysts — is the most common genetic cause of kidney failure. PKD genes encode proteins that either localize in primary cilia or regulate ciliary proteins. The genes most often changed in patients with PKD are PKD1 and PKD2, encoding polycystin-1 and polycystin-2, respectively. Polycystin-1 and polycystin-2 form a complex that localizes in primary cilia and functions as a cation channel.
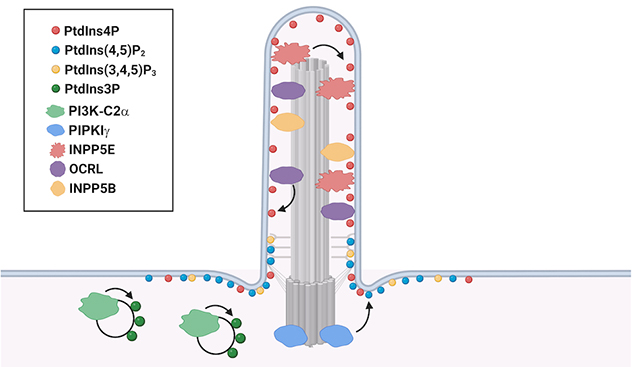 Localization of phosphoinositides with associated phosphatases and kinases in primary cilia
Localization of phosphoinositides with associated phosphatases and kinases in primary cilia
The unique enrichment of specific phosphoinositide species in different ciliary compartments is generated by the specific phosphatases and kinases.
Accumulating evidence suggests that the level of functional polycystin complex in primary cilia is associated with the progression and severity of renal cystogenesis. Importantly, restoration of functional polycystins in cilia suppresses and even reverses the disease in PKD mouse models. Our work suggests that a unique cilium-specific phosphoinositide signaling axis regulates the ciliary entry and homeostasis of polycystins. Our team's current efforts are focused on dissecting the underlying molecular mechanism and discovering molecules that can be targeted to restore the appropriate level of polycystin complex in cilia in the context of PKD.
Skeletomuscular development and disease
The skeleton muscle, bone and fat tissues are differentiated from the same type of progenitor cells. Using conditional PIPKIγ knockout mice, our team found that PIPKIγ not only participates in the differentiation of osteoblasts and adipocytes from progenitor cells but also is required for the maintenance and growth of skeletal muscle by regulating mitochondria and lipid droplets.
Among the three PtdIns4P-5-kinases, PIPKIγ is the only one that causes severe developmental defects and lethality when knocked out in mice. Moreover, a missense mutation in PIP5K1C that encodes a kinase-dead PIPKIγ is associated with lethal congenital contractural syndrome type 3 (LCCS3), which is embryonic lethal and exhibits severe muscle waste. The goal of our study is to understand how PIPKIγ and PtdIns(4,5)P2 regulate the differentiation, growth and homeostasis of bone, muscle and fat tissues.
 PIPKIγ and the ciliopathy protein HYLS1 form a complex to produce PtdIns(4,5)P2 at the ciliary base.
PIPKIγ and the ciliopathy protein HYLS1 form a complex to produce PtdIns(4,5)P2 at the ciliary base.
The specific PtdIns(4,5)P2 pool at the ciliary base is essential for the guided transportation of ciliary proteins into the primary cilium.