-
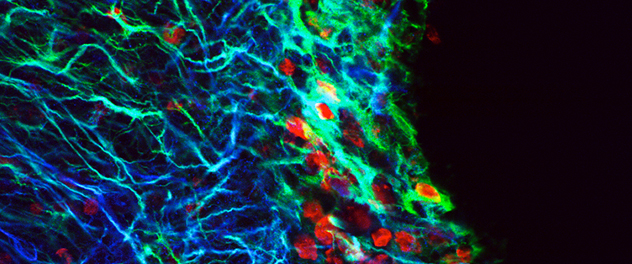
Overcoming pitfalls
Until recently, thymidine analogs were used to label stem cells for experimental implantation into the rodent brain. Results suggested extraordinary abilities of adult stem cells. However, a high percentage of cells typically die shortly after transplantation. Dr. Burns and colleagues found that thymidine analog (red) from dead cells can be recycled and incorporated into the DNA of live proliferating cells in the recipient brain, making them appear as transplanted cells. As such, this technique is now known to be unreliable.
-
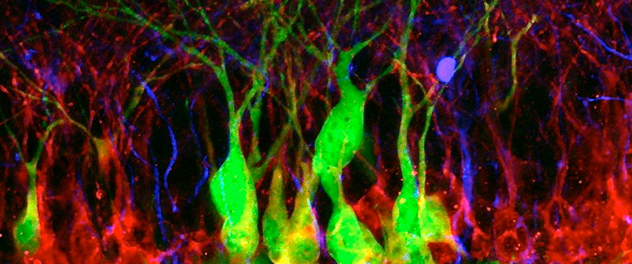
Transplanting plasticity
Hippocampal neurogenesis is involved in learning and memory, but declines with age, brain radiation and neurodegeneration, suggesting a possible role for stem cell therapies in these diseases. Here, transplanted embryonic stem cell-derived cells (green) incorporate alongside other newly born doublecortin-expressing (red) neurons in the hippocampal dentate gyrus.
-
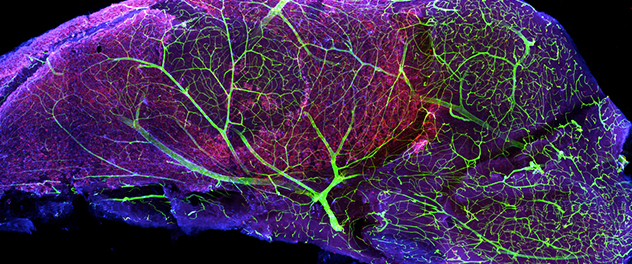
A vascular niche for neurogenesis
The subventricular zone (SVZ) is one of the major sources of adult-born neurons. Blood vessels in the SVZ help regulate the behavior of neural stem cells, providing an important vascular niche for neurogenesis. This image of a microdissected mouse SVZ stained with nestin (red), glial fibrillary acidic protein (blue) and laminin — a vascular basement membrane protein (green) — demonstrates the intricate vascular network in the SVZ.
-
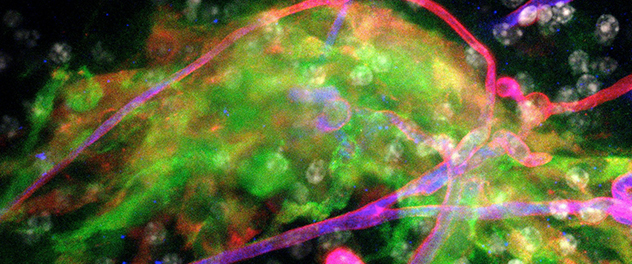
Niche tropism
Co-culturing neural stem cells with explants of neurogenic regions facilitates research on neural stem cell interactions with neurogenic regions. Here, neural stem cells (green) are attracted to a cluster of blood vessels in the neurogenic subventricular zone.
-
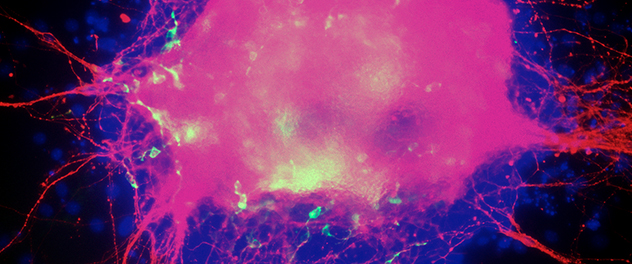
Differentiating embryonic stem cell colony
When encouraged to differentiate toward a neuronal lineage, embryonic stem cells recapitulate the early stages of embryonic development. Here, proliferation of neural progenitors gave rise to a large number of neurons (red), prior to appearance of astrocytes (green).
Overview
The Regenerative Neurosurgery and Neuro-Oncology Lab of Terry C. Burns, M.D., Ph.D., serves as a translational hub for neuroregenerative therapies across Mayo Clinic. In collaboration with colleagues at multiple institutions across the country and internationally, Dr. Burns' goal is to expedite the development, rigorous evaluation and translation of safe and effective regenerative therapies for ischemic, traumatic, degenerative and neoplastic diseases of the central nervous system.
Applying neuroregenerative research across neurological disorders
Many mechanisms of DNA damage, senescence, neuroinflammation and altered stem cell function are common to brain tumors, neurodegeneration and central nervous system injury. Therefore, the Mayo Clinic Regenerative Neurosurgery and Neuro-Oncology Lab takes a broad approach to neuroregenerative research, focusing on commonalities that may improve quality of life for patients with a range of neurological disorders.
For example, in the effort to stop tumor cell proliferation, treatments for brain tumors often induce further DNA damage and inflammation, impeding cognitive outcomes in patients who undergo long-term whole-brain radiation. The Regenerative Neurosurgery and Neuro-Oncology Lab is focused on developing and translating regenerative therapies that improve innate defenses against brain tumors, degenerative processes and neurological injuries, thereby improving neurological function and quality of life.
Focusing on cures for humans, not mice
At present, most therapies are developed and tested in mice prior to human clinical trials. Unfortunately, the hundreds of promising therapies developed and tested in rodent models of Alzheimer's disease, stroke, traumatic brain injury and spinal cord injury, among others, have failed when ultimately tested in human clinical trials.
Dr. Burns' recent work indicates that the molecular mechanisms underlying mouse models of neurological disease may be profoundly different from those of human patients. Neurosurgical access to the human brain provides a critical window into mechanisms of human disease that can help tailor ongoing efforts toward treatment of neurological disease in humans, rather than mice.
Dr. Burns is working with an interdisciplinary team of scientists, engineers and clinicians to collaboratively develop an integrated neuroregenerative pipeline focused specifically on human patients with neurological diseases.
More about neuroregeneration research
Listen to Dr. Burns discuss neuroregeneration research in an episode of NCTalks podcast on the Neuro Central website.
View Dr. Burns' biography to learn more about his background and research interests.