 Immune responses impact brain function
Immune responses impact brain function
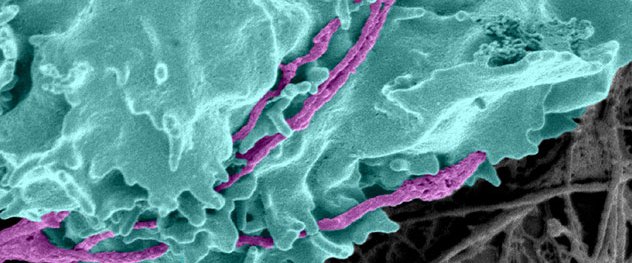
An anti-axonal cytotoxic T cell (turquoise) attacks immunologically stressed axons (purple). Evidence from the Translational Neuroimmunology Lab indicates that the cytotoxic molecules perforin and granzyme are introduced, rapidly and irrevocably damaging the axons.
Overview
The Translational Neuroimmunology Laboratory of Charles L. Howe, Ph.D., focuses on understanding and therapeutically manipulating immunological responses to injury, loss of homeostasis, degeneration, autoimmunity and infection in the central nervous system, with the goal of protecting neurons, axons and neural circuits.
The lab's long-term research goal is to develop novel therapeutic strategies to protect and repair the central nervous system. To achieve this goal, Dr. Howe's research team manipulates and modulates the immune response and targets the injury pathways and dyshomeostatic responses induced by neuroinflammation.
The key element of functional recovery after neurological insult in children and adults is preservation and protection of axons and normalization of synaptic connections and neural circuits. Loss of axons and synapses means loss of information transfer through the neural axis and failure to maintain motor, sensory or cognitive function.
The lab uses a unique repertoire of tools, therapeutic agents and model systems to identify and characterize cells, molecules and signals that are involved in shaping the inflammatory environment in the central nervous system during neurological insult.
Tools utilized include high-resolution 4D microscopy, flow cytometry, longitudinal immunophenotyping, EEG, behavioral analyses, reporter mice that are genetically deficient in specific chemokine receptors, and mice that are genetically engineered to express neoantigens and novel T cell receptors. New directions in the lab include the production and interrogation of human stem cell-derived neurons and glia and analysis of patient-specific immune effectors.
If physicians can preserve even a subset of axons and synaptic connections during chronic demyelination, inflammation, tissue remodeling, scarring and healing, or during the immediate aftermath of seizures, spinal cord injury, trauma or stroke, then patients will maintain a base for restorative, regenerative and prosthetic interventions and other therapeutic strategies aimed at renormalizing neurological structure and function.
About Dr. Howe
In addition to running the Translational Neuroimmunology Lab, Dr. Howe holds several leadership positions at Mayo Clinic. Dr. Howe is a professor of neurology and an associate professor of neuroscience at Mayo Clinic College of Medicine and Science in Rochester, Minnesota. He also is chair of the Division of Experimental Neurology in the Department of Neurology, director of the Office of Core Shared Services and director of research for the Center for Multiple Sclerosis and Autoimmune Neurology.