-
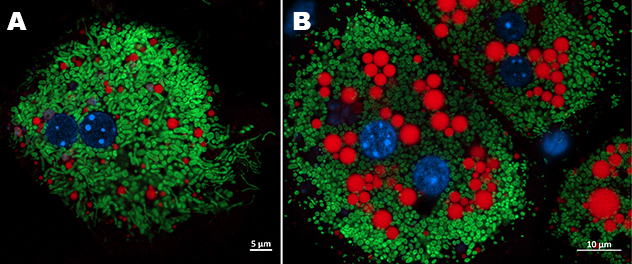
Mitochondrial-lipid droplets interaction in hepatocyte under lipotoxic stress
Primary mouse hepatocytes from a control mouse (A) and a MASH mouse (B) were stained with MitoTracker (green) to visualize mitochondria and LipidTox (red) to label lipid droplets. Nuclei were counterstained with Hoechst (blue).
-
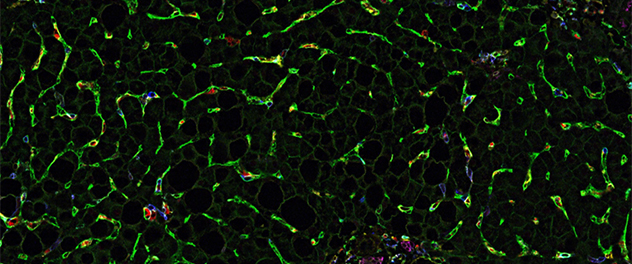
Myeloid cells-LSEC adhesion in MASH
Co-detection by indexing (CODEX) multiplexed imaging of this liver biopsy image from a patient with metabolic dysfunction-associated steatohepatitis (MASH) shows numerous myeloid cells adhering to liver sinusoidal endothelial cells (LSECs).
-
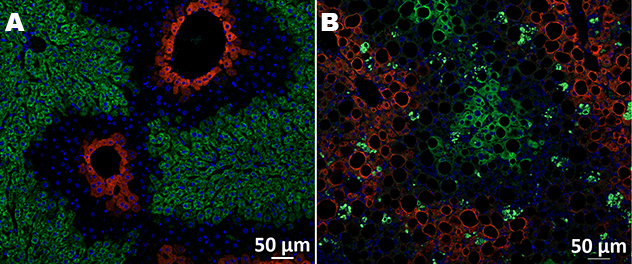
Zonal distribution of steatosis in MASH
This image shows a liver histological section from a normal control mouse (A) and a mouse with diet-induced metabolic dysfunction-associated steatohepatitis (MASH) (B) for zonal quantification of steatosis. Glutamine synthetase (GS) (red) delineates the pericentral area, and CYP2F2 (green) delineates the periportal area (CV, central vein, and PV, portal vein).
Overview
With the epidemic of obesity, metabolic dysfunction-associated steatohepatitis (MASH) has become a significant public health problem with limited therapeutic options.
The Lipotoxicity and Liver Inflammation Laboratory led by Samar H. Ibrahim, M.B., Ch.B., investigates mechanisms of liver injury and inflammation in MASH. The research team is highly collaborative and dynamic.
The goal of Dr. Ibrahim's work is to better understand the cellular and molecular mechanisms involved in MASH pathogenesis and identify mechanism-based therapeutic targets to prevent MASH progression to irreversible end-stage liver disease. Dr. Ibrahim's lab also has examined the role of an obesity-induced diet introduced at the time of conception in nutritional reprogramming of the offspring epigenome, leading to an accentuated murine MASH phenotype. The long-term goal of this line of work is to identify epigenetic modifiers as potential therapeutic targets for a subset of patients with early-onset MASH.
Studies
The Ibrahim lab has defined the pathogenetic role of different stress kinases, including glycogen synthase kinase (GSK) 3β and mixed lineage kinase 3 in murine MASH. These studies have shown that blocking these kinases has beneficial effects in in vitro and in vivo MASH models.
Recent work in the laboratory has focused on:
- Defining the mechanisms of lipotoxic endotheliopathy and identifying liver sinusoidal endothelial cell (LSEC)-based therapeutic approaches in MASH.
- Identifying the molecular mediators and novel therapeutic target of lipotoxicity-induced ferroptosis in MASH.
- Examining the role of hepatocyte and LSEC mitochondrial dysfunction in the pro-inflammatory response in MASH.
Learn more about the focus areas of Dr. Ibrahim's lab.